Calcium Chloride and Water Equation
CaC 2 N 2 CaCN 2 C. Oiling can either slow down the process or.

Chemical Reactions And Equation Cbse Class 10 Science Ncert Solutions Maths Ncert Solutions Science Textbook Chemical Reactions
We can generate a second equation however by noting that one Ag ion is released for every Br-ion.

. It is represented by using the below equation. If it gets warmer more ice becomes water. The optimum conditions for NaOH CO 2 flowrate and salinity for the process were found to be 5 gL 2 Lmin and 75 gL respectively.
A multistage process for extracting minerals from desalination reject brine while concurrently capturing CO 2 was evaluated. CO 2 uptake and calcium recovery were optimized using Response Surface Methodology. In the equation above 50000 represents the equivalent weight of CaCO3 50 multiplied by 1000 mg.
Uses of Calcium phosphate Ca 3 PO 4 2. K sp Ag Br- 50 x 10-13. When alkalinity is reported it is expressed as calcium carbonate or CaCO3-.
P ΔH ΔQ ΔV. What is rusting of iron with. What if the sample used in the test above was distilled water.
The drying agent used in drying hydrogen chloride gas is conc. If there is any moisture in the air anhydrous calcium chloride will absorb it. The balanced equation for the reaction.
Rusting of iron is the process when iron combines with water and oxygen to generate hydrated iron. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. 216 Reaction of a Non-metallic Oxide with Base You saw the reaction between carbon dioxide and calcium hydroxide lime water in Activity 25.
The use of calcium chloride cuts out the water vapour in the air and prevents rusting. Calcium sulfate or calcium sulphate is the inorganic compound with the formula CaSO 4 and related hydratesIn the form of γ-anhydrite the anhydrous form it is used as a desiccantOne particular hydrate is better known as plaster of Paris and another occurs naturally as the mineral gypsumIt has many uses in industry. Hydrogen chloride gas is highly soluble in water.
Phosphorous pentoxide and calcium oxide are good drying agents but they cannot be used to dry hydrogen chloride gas because they react with hydrogen chloride. It is used in natural farming. It is also hydrolyzed to cyanamide H 2 NCN.
However an online Chemical Equation Balancer Calculator will provide you the balanced equation equilibrium constant with chemical name and formula of all reactants and product of a chemical equation. Water soluble calcium phosphate is an important material for plant growth and is commonly dispersed in the soil. Calcium hydroxide which is a base reacts.
Fifty thousand is a constant used in the formula. We then write the solubility product expression for this reaction. Commonly the term called nitrolime which is a calcium cyanamide can be used as fertilizer.
The most effective prevention tool is galvanization which prevents water and air from reacting with the metal substance. Structure of Ca 3 PO 4 2. The equation for the formation of rust is.
The measurement of alkalinity of water is necessary to determine the amount of lime and soda needed for water softening eg for corrosion control in conditioning the boiler feed water. The list of uses of calcium carbonate is given below. Calcium carbide compound reacts with nitrogen at higher temperatures to produce calcium cyanamide.
100 removal of Ca. One equation cant be solved for two unknowns the Ag and Br-ion concentrations. Sodium chloride is the salt.
By making considerable change in enthalpy equation we get. Metallic oxides react with acids to give salts and water similar to the reaction of a base with an acid metallic oxides are said to be basic oxides. Calcium carbonate can also be an additive to food products for livestock animals and humans and as a supplement in vitamins.
P 7 41 11. Calcium phosphate may dissolve slightly in CO 2-containing water. Calcium phosphate is insoluble in water but soluble in acids.
Allow these test tubes to sit for a few days before. Because there is no other source of either ion in this solution the concentrations of these ions. In water and sewer treatment plants calcium carbonate is employed in the removal of acidity and impurities.
All forms are white solids that are poorly soluble in water. If water contents are not acceptable water can be added provided the water will not drain out of the soil quickly Schmall and Braun 2006. When the ionic compound salt is added to the equation it lowers the freezing point of the water which means the ice on the ground cant freeze that layer of water at 32 F anymore.
The water however can still melt the ice at that temperature which results in less ice on the roads. In addition to being applicable to the entire range of soils it is also applicable to difficult ground conditions including large boulders and cobbles or debris-rich non-engineered fills. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
Alkalinity of water is mainly caused by the presence of hydroxide ions OH bicarbonate ions HCO 3 and carbonate ions CO 3 2 or a mixture of two of these ions in.
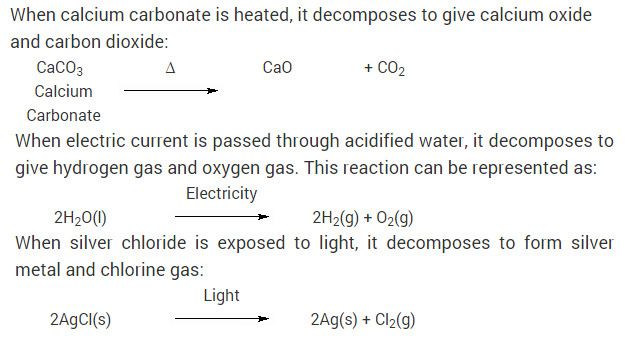
Chemical Reactions And Equation Cbse Class 10 Science Ncert Solutions Chemical Reactions Science Textbook Science

Calcium Chloride Baking Soda Water Reaction Calcium Chloride Baking Soda Baking

Pin By Carin Barber On Chemistry Notes Chemistry Notes Chemistry Math

Solubility Product Constants Silver Chloride Agcl Is Rather Insoluble In Water Careful Experiments Show That If Solid Solubility Experiments Silver Chloride
No comments for "Calcium Chloride and Water Equation"
Post a Comment